Annual product quality review ppt North Katoomba

Annual/Product Don’t miss Quality Review this course An organized and comprehensive review of all production, analytical, stability, complaints, changes, deviations, recalls and customer data associated with a pharmaceutical product so as to monitor the drug product quality and improve where necessary. By- Prashant S Mengshetti mengshettips@gmail.com
Annual Product Review prashant-mengshetti.webs.com
Preparation of Annual Product Review (APR. Toprovide guidance to industry on how to implement Product Quality Reviews (PQRs). 3. Scope PQRsare a requirementin PIC/S Guide for GMP, Clause 1.4. Regularperiodic or rolling quality reviews of all registered pharmaceuticalproducts, including exportВonlyproducts, should be conducted to highlight any overall trends (not necessarily visible, So, if your result is 2 (a six sigma process), you are occupying 50% of your process specifications and youВґre less prone to non-complying product. If yuor result is 1, you are occupying 100% of your specifications, so any variation from the mean of your process would result in a fraction of non-complying product..
Product Quality Management Annual Product Review (APR/PQR) process •Implementation of “better” assay for other commercial products •Assess suitability of assay •Determine if options Title: Annual Product Review Author: https://www.gmpsop.com Subject: This procedure provides a guideline to annual product review which is required to be performed for each product produced for the commercial market to evaluate data, trends and to identify any preventative or corrective action that would lead to product qu\ ality improvements and report them to management.
Toprovide guidance to industry on how to implement Product Quality Reviews (PQRs). 3. Scope PQRsare a requirementin PIC/S Guide for GMP, Clause 1.4. Regularperiodic or rolling quality reviews of all registered pharmaceuticalproducts, including exportВonlyproducts, should be conducted to highlight any overall trends (not necessarily visible Both parts of the EU-GMP Guidelines require the Product Quality Review (PQR) to verify the consistency and appropriateness of existing processes, but also to identify product and process improvement opportunities. The FDA 21CFR 211 requires an Annual Product Review (APR) to evaluate annually the quality standards of each drug product.
Annual Product Quality Review (APQR / PQR) Annual product quality review shall be evaluated and recorded, annually for specific drug substance or drug product to verifying the consistency of the manufacturing process. Annual Product Quality Review (APQR / PQR) Annual product quality review shall be evaluated and recorded, annually for specific drug substance or drug product to verifying the consistency of the manufacturing process.
SOP for Annual Product Quality Review Purpose: - This SOP gives the method of collecting data for Annual product review Responsibility:-Q. A. ManagerPrecautions: Not applicable General Condition: Annual Product review of a finished is prepared for all the batches manufactured in a year i.e. January month to December month. Product Quality Review?Product Quality Review is regular periodic or rolling quality reviews of all licensed medicinal products, including export only products, which are conducted with the objective of verifying the consistency of the existing process, the appropriateness of current specifications for both starting materials and finished
Finally, we will discuss the expectation for product, process, and quality system review to support the evolving regulatory expectation for continuous improvement within a contemporary Pharmaceutical Quality System. Learning Benefits:-Requirements for Annual Product Reviews (Product Quality Reviews). -Annual Product Reviews and Annual Reports. So, if your result is 2 (a six sigma process), you are occupying 50% of your process specifications and youВґre less prone to non-complying product. If yuor result is 1, you are occupying 100% of your specifications, so any variation from the mean of your process would result in a fraction of non-complying product.
Periodic Product Quality Reviews or Annual Product Reviews of drug products are required by the U.S., EU and Canada. If used effectively, these reviews can be a powerful Quality Assurance tool. The Product Quality Review Process includes: Process Performance and Product Quality Monitoring, Corrective Action/Preventive Action, Change Management A. Executive Summary Marketing Authorization Recalls, Field Alerts, and Safety Notifications Exception Report Summary A. Executive Summary B. Technical Agreements C. Incoming Materials D. Starting/ Packaging Materials Review E. Vendor / Supplier Assurance Equipment Utilization
Objective :To lay down a procedure to conduct Annual Product Quality Review for all pharmaceutical products. Scope :This Standard Operating Procedure is applicable for all products manufactured at formulation plant of Pharmaceuticals Company (Name). Responsibility Officer / Executive – QA shall We will be posting product reviews ranging from tech products, to household products. We will also be trying in a variety of services that we use and share our experiences with you. We hope that 365 days after, apqr.org will be your top- resources for all the product / service reviews that you might need.
Annual Product Quality Review (APQR / PQR) Annual product quality review shall be evaluated and recorded, annually for specific drug substance or drug product to verifying the consistency of the manufacturing process. Periodic Product Quality Reviews or Annual Product Reviews of drug products are required by the U.S., EU and Canada. If used effectively, these reviews can be a powerful Quality Assurance tool. The Product Quality Review Process includes: Process Performance and Product Quality Monitoring, Corrective Action/Preventive Action, Change Management
Annual Product Review - GMP SOP Standard Operation Procedure 1. Regulatory Reference2. Purpose3. Scope4. Responsibilities and Accountabilities4.1 Quality Assurance4.2 Production4.3 Quality Control4.4 Regularly Affairs and Clinical Safety Home; The page is under construction!
– At least annual product quality reviews – Management reviews – Supplier qualification and ongoing monitoring – Internal audits . 7 . Maturity of QM programs • Leading vs. lagging As the end of the year is approaching, you may be involved in preparing a business review presentation. Here’re some simple tips on how to make it visually attractive. How to make financial results more interesting, inspirations for showing sales, production, accounting data and other KPIs in eye-catching visual form. I was involved in a … Continue reading How to make attractive Annual
Annual Product Review Developing an SOP

SOP on Annual Product Review of Drug Product Quality. 02/03/2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. There are significant differences between the United States and European Union requirements for the annual review of records related to the manufacturing and control for pharmaceutical products and active pharmaceutical ingredients. Mar 02, 2008. By Pharmaceutical Technology Editors . Pharmaceutical …, Annual Product Review (APR) as GMP Deviation in FDA Warning Letters Register now for ECA's GMP Newsletter Since 2002, CONCEPT HEIDELBERG (a service provider entrusted by the ECA Foundation) has been analysing annually FDA's Warning Letters to drug and API manufacturers..
ANNUAL PRODUCT QUALITY REVIEW REGULATORY ASPECT

(PDF) ANNUAL PRODUCT REVIEWS HOW TO CONDUCT AN. 02/03/2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. There are significant differences between the United States and European Union requirements for the annual review of records related to the manufacturing and control for pharmaceutical products and active pharmaceutical ingredients. Mar 02, 2008. By Pharmaceutical Technology Editors . Pharmaceutical … https://en.wikipedia.org/wiki/Information_quality So, if your result is 2 (a six sigma process), you are occupying 50% of your process specifications and you´re less prone to non-complying product. If yuor result is 1, you are occupying 100% of your specifications, so any variation from the mean of your process would result in a fraction of non-complying product..

PowerPoint template for yearly company performance review. various company areas review diagram: goals benchmarks and completion checklist, events month by month, financial summary, KPIs 25 flat icons: turnover and revenue, profit, costs, product, employment, production, sales The PPT package contains: Goals review presentation section “APQR is a Annual Product Quality Review somewhere known as APR (Annual Product Review)” APQR contains a documented evidence oriented review of all activities related to a product manufactured in a organization, it covers all parameters which affects a product quality from Manufacturing stage to market performance.
efficient annual product quality review. This written procedure will provide the critical information and details to guide the participant in the preparation of a company SOP on product annual review. Each participant will also receive an example of a product annual review report that outlines the necessary information to meet 31/07/2019В В· 5.6 Changes proposed, approved and implemented that are directly or indirectly related to the product, in-case if a change control is raised related to a multi-product facility should be mentioned in the annual product review report of all the products that are manufactured in the facility.
Finally, we will discuss the expectation for product, process, and quality system review to support the evolving regulatory expectation for continuous improvement within a contemporary Pharmaceutical Quality System. Learning Benefits:-Requirements for Annual Product Reviews (Product Quality Reviews). -Annual Product Reviews and Annual Reports. ‚systematic application of quality mgt policies, procedures and practices to the tasks of assessing, controlling, communicating and review of risks†[dtto.] Product Lifecycle : ‚all phases in the life of the product from initial development through marketing until the productвЂs discontinuation†[dtto.]
Requirement of APQR• In USA - "Annual Product Review“• In Europe, the EU GMP Guideline uses the term "Product Quality Review".• Requirement or expectations are almost same• APQR should be conducted for all commercial product.• APQR should confirm the State of Control 10. Objective :To lay down a procedure to conduct Annual Product Quality Review for all pharmaceutical products. Scope :This Standard Operating Procedure is applicable for all products manufactured at formulation plant of Pharmaceuticals Company (Name). Responsibility Officer / Executive – QA shall
23/11/2012 · PowerPoint Presentation: Product annual/ quality review The us food and drug administration proposed the requirement in feb13' 1976 rewriting of the GMP’s The purpose was to provide reliable procedure for a drug manufacturer to review the quality standard 3 QUALITY ASSUARANCE Saturday, 14 September 2013. SOP FOR ANNUAL PRODUCT REVIEW I. PURPOSE. To define a procedure to perform Annual Product Review of finished APIs and Intermediates. II. SCOPE. The SOP is applicable for all finished APIs and Intermediates manufactured in, Unit – V. III.
OBJECTIVE : To establish a procedure for the preparation, review and approval of Annual product reviews to assure the consistent and acceptable quality of each product manufactured for distribution and apprise upper management of any changes needed. RESPONSIBILITY : Officer - Quality Assurance to … Requirement of APQR• In USA - "Annual Product Review“• In Europe, the EU GMP Guideline uses the term "Product Quality Review".• Requirement or expectations are almost same• APQR should be conducted for all commercial product.• APQR should confirm the State of Control 10.
PowerPoint template for yearly company performance review. various company areas review diagram: goals benchmarks and completion checklist, events month by month, financial summary, KPIs 25 flat icons: turnover and revenue, profit, costs, product, employment, production, sales The PPT package contains: Goals review presentation section Annual Product Review - GMP SOP Standard Operation Procedure 1. Regulatory Reference2. Purpose3. Scope4. Responsibilities and Accountabilities4.1 Quality Assurance4.2 Production4.3 Quality Control4.4 Regularly Affairs and Clinical Safety
How to Conduct an Effective Annual Product Quality Review Duration: 60 Minutes This webinar will give a brief overview of the general procedure for the preparation and documentation of the Annual Product Quality Review and also focuses on the regulations and the … Welcome to Pharmaprojects’ 2017 review of trends in pharmaceutical R&D. For around a quarter of a century now, I’ve been taking an annual look at the evolution of pharma R&D, and in this article, I’ll look at how the land lies at the start of 2017. We’ll assess industry trends by examining the pipeline
Description. 1.0 introduction. annual product quality review is prepared in compliance with cgmp requirements. they are crucial part of good manufacturing practices and also quality management of a pharmaceutical product. the quality review process is done annually to evaluate the quality standards of the ongoing process and also the specifications or control procedures. it is given for every Annual product quality reviews helps to ascertain the integrity of quality of product and the process and controlls, it helps in further improvement of quality of pharmaceutical product manufactured in a firm. Annual product quality reviews APQR should also recommend any changes if required so as to improve the quality of product.
Requirement of APQR• In USA - "Annual Product Review“• In Europe, the EU GMP Guideline uses the term "Product Quality Review".• Requirement or expectations are almost same• APQR should be conducted for all commercial product.• APQR should confirm the State of Control 10. Annual Quality Review July 2014 - Annual Quality Review July 2014 Agenda Welcome and introduction Qualification and Development specific review, GIFBuddy particular review and bonus, GIFBuddy review, GIFBuddy review biggest bonus, GIFBuddy review demo product, GIFBuddy review demo in action PowerPoint PPT presentation free to view . Page of . CrystalGraphics. Home About Us Terms and

Toprovide guidance to industry on how to implement Product Quality Reviews (PQRs). 3. Scope PQRsare a requirementin PIC/S Guide for GMP, Clause 1.4. Regularperiodic or rolling quality reviews of all registered pharmaceuticalproducts, including exportВonlyproducts, should be conducted to highlight any overall trends (not necessarily visible Title: Annual Product Review Author: https://www.gmpsop.com Subject: This procedure provides a guideline to annual product review which is required to be performed for each product produced for the commercial market to evaluate data, trends and to identify any preventative or corrective action that would lead to product qu\ ality improvements and report them to management.
Annual Product Review Developing an SOP

Annual Product Quality Review (APQR) Pharma Pathway. 13/06/2014 · PowerPoint Presentation: Annual Product Quality Review(APQR) Annual Product Quality Review (APQR) must be conducted for each commercial product. An Annual schedule for completing APQR shall be prepared by Manager QA. This schedule shall established the specific product to be reviewed and the “cut off ” date for each APR Shall be, ANNUAL PRODUCT QUALITY REVIEW: REGULATORY ASPECT INTRODUCTION: Annual Product Quality Review:1, 2, 3 Annual product review is an evaluation conducted annually to assess the quality standard of each drug product with the view to verify the consistency of existing process and to check the appropriateness of current specifications and to highlight any tends in order to determine the need to.
The Annual Product Quality Review
SOP on Annual Product Review of Drug Product Quality. Product Quality Review?Product Quality Review is regular periodic or rolling quality reviews of all licensed medicinal products, including export only products, which are conducted with the objective of verifying the consistency of the existing process, the appropriateness of current specifications for both starting materials and finished, – At least annual product quality reviews – Management reviews – Supplier qualification and ongoing monitoring – Internal audits . 7 . Maturity of QM programs • Leading vs. lagging.
So, if your result is 2 (a six sigma process), you are occupying 50% of your process specifications and youВґre less prone to non-complying product. If yuor result is 1, you are occupying 100% of your specifications, so any variation from the mean of your process would result in a fraction of non-complying product. Both parts of the EU-GMP Guidelines require the Product Quality Review (PQR) to verify the consistency and appropriateness of existing processes, but also to identify product and process improvement opportunities. The FDA 21CFR 211 requires an Annual Product Review (APR) to evaluate annually the quality standards of each drug product.
Toprovide guidance to industry on how to implement Product Quality Reviews (PQRs). 3. Scope PQRsare a requirementin PIC/S Guide for GMP, Clause 1.4. Regularperiodic or rolling quality reviews of all registered pharmaceuticalproducts, including exportВonlyproducts, should be conducted to highlight any overall trends (not necessarily visible Annual Quality Review July 2014 - Annual Quality Review July 2014 Agenda Welcome and Quality Review Is Mandatory - 4491-32 Quality Review of Tax Return v1.0 VO.ppt Quality Review Is Mandatory Pub 4012 Tab 13 Form 13614-C Section C LEVEL 2 TOPIC 11/30/2010 * NJ Training TY2010 v1.0 PowerPoint PPT presentation free to view . Quality Sleep with Leesa Mattress Reviews - Leesa …
Download a .PDF of this Case Study here: Managed Service Solution for Annual Product Review (APR) Client Business Situation. Client was experiencing multiple problems with its Annual Product Review (APR) process for its externally manufactured products, which included OTC Pharmaceuticals, Cosmetics, and Medical Devices (See Note). A. Executive Summary Marketing Authorization Recalls, Field Alerts, and Safety Notifications Exception Report Summary A. Executive Summary B. Technical Agreements C. Incoming Materials D. Starting/ Packaging Materials Review E. Vendor / Supplier Assurance Equipment Utilization
Annual Product Quality Review (APQR / PQR) Annual product quality review shall be evaluated and recorded, annually for specific drug substance or drug product to verifying the consistency of the manufacturing process. Periodic Product Quality Reviews or Annual Product Reviews of drug products are required by the U.S., EU and Canada. If used effectively, these reviews can be a powerful Quality Assurance tool. The Product Quality Review Process includes: Process Performance and Product Quality Monitoring, Corrective Action/Preventive Action, Change Management
Annual Product Quality Review (APR) is an evaluation conducted annually to determine if there are any possible changes in the process or manufacturing of the pharmaceutical product or any change in the specifications of the product or any change in the manufacturing process. It is designed to minimize the product defects and also the risks 23/11/2012 · PowerPoint Presentation: Product annual/ quality review The us food and drug administration proposed the requirement in feb13' 1976 rewriting of the GMP’s The purpose was to provide reliable procedure for a drug manufacturer to review the quality standard 3
“APQR is a Annual Product Quality Review somewhere known as APR (Annual Product Review)” APQR contains a documented evidence oriented review of all activities related to a product manufactured in a organization, it covers all parameters which affects a product quality from Manufacturing stage to market performance. quality product. •EU Inspectors expect discussion & evaluation of the data presented and ID of appropriate process improvements as required •The FDA “Annual product review” is intended to confirm that every batch of product released during the review …
23/11/2012 · PowerPoint Presentation: Product annual/ quality review The us food and drug administration proposed the requirement in feb13' 1976 rewriting of the GMP’s The purpose was to provide reliable procedure for a drug manufacturer to review the quality standard 3 conformance with Title 10 Section 425.22 (a) Quality improvement. An annual product review (APR) should be conducted for every commercial product. The purpose of this review is to verify the consistency of the manufacturing process. An annual product review (APR) should be conducted for every commercial product. The purpose of this
We will be posting product reviews ranging from tech products, to household products. We will also be trying in a variety of services that we use and share our experiences with you. We hope that 365 days after, apqr.org will be your top- resources for all the product / service reviews that you might need. How to Conduct an Effective Annual Product Quality Review Duration: 60 Minutes This webinar will give a brief overview of the general procedure for the preparation and documentation of the Annual Product Quality Review and also focuses on the regulations and the …
efficient annual product quality review. This written procedure will provide the critical information and details to guide the participant in the preparation of a company SOP on product annual review. Each participant will also receive an example of a product annual review report that outlines the necessary information to meet Requirement of APQR• In USA - "Annual Product Review“• In Europe, the EU GMP Guideline uses the term "Product Quality Review".• Requirement or expectations are almost same• APQR should be conducted for all commercial product.• APQR should confirm the State of Control 10.
Annual Product Quality Review is prepared in pharmaceutical to review the consistency of the products annually regarding their quality including the deviations, change controls and market complaints.It is used as an effective product quality improvement tool. Finally, we will discuss the expectation for product, process, and quality system review to support the evolving regulatory expectation for continuous improvement within a contemporary Pharmaceutical Quality System. Learning Benefits:-Requirements for Annual Product Reviews (Product Quality Reviews). -Annual Product Reviews and Annual Reports.
GMP- APQR training SlideShare
PPT – Annual Review PowerPoint presentation free to view. QUALITY ASSUARANCE Saturday, 14 September 2013. SOP FOR ANNUAL PRODUCT REVIEW I. PURPOSE. To define a procedure to perform Annual Product Review of finished APIs and Intermediates. II. SCOPE. The SOP is applicable for all finished APIs and Intermediates manufactured in, Unit – V. III., quality product. •EU Inspectors expect discussion & evaluation of the data presented and ID of appropriate process improvements as required •The FDA “Annual product review” is intended to confirm that every batch of product released during the review ….
The Annual Product Quality Review. Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article (PDF Available) · February 2012 with 4,656 Reads How we measure 'reads' A 'read' is counted each time, Welcome to Pharmaprojects’ 2017 review of trends in pharmaceutical R&D. For around a quarter of a century now, I’ve been taking an annual look at the evolution of pharma R&D, and in this article, I’ll look at how the land lies at the start of 2017. We’ll assess industry trends by examining the pipeline.
Guidance for Industry

Annual Quality Report ahcancal.org. Product Quality Review?Product Quality Review is regular periodic or rolling quality reviews of all licensed medicinal products, including export only products, which are conducted with the objective of verifying the consistency of the existing process, the appropriateness of current specifications for both starting materials and finished https://en.wikipedia.org/wiki/Advanced_Product_Quality_Planning OBJECTIVE : To establish a procedure for the preparation, review and approval of Annual product reviews to assure the consistent and acceptable quality of each product manufactured for distribution and apprise upper management of any changes needed. RESPONSIBILITY : Officer - Quality Assurance to ….

02/03/2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. There are significant differences between the United States and European Union requirements for the annual review of records related to the manufacturing and control for pharmaceutical products and active pharmaceutical ingredients. Mar 02, 2008. By Pharmaceutical Technology Editors . Pharmaceutical … So, if your result is 2 (a six sigma process), you are occupying 50% of your process specifications and you´re less prone to non-complying product. If yuor result is 1, you are occupying 100% of your specifications, so any variation from the mean of your process would result in a fraction of non-complying product.
We will be posting product reviews ranging from tech products, to household products. We will also be trying in a variety of services that we use and share our experiences with you. We hope that 365 days after, apqr.org will be your top- resources for all the product / service reviews that you might need. As the end of the year is approaching, you may be involved in preparing a business review presentation. Here’re some simple tips on how to make it visually attractive. How to make financial results more interesting, inspirations for showing sales, production, accounting data and other KPIs in eye-catching visual form. I was involved in a … Continue reading How to make attractive Annual
Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article (PDF Available) · February 2012 with 4,656 Reads How we measure 'reads' A 'read' is counted each time 23/11/2012 · PowerPoint Presentation: Product annual/ quality review The us food and drug administration proposed the requirement in feb13' 1976 rewriting of the GMP’s The purpose was to provide reliable procedure for a drug manufacturer to review the quality standard 3
Annual Product Review (APR) as GMP Deviation in FDA Warning Letters Register now for ECA's GMP Newsletter Since 2002, CONCEPT HEIDELBERG (a service provider entrusted by the ECA Foundation) has been analysing annually FDA's Warning Letters to drug and API manufacturers. Annual Quality Review July 2014 - Annual Quality Review July 2014 Agenda Welcome and Quality Review Is Mandatory - 4491-32 Quality Review of Tax Return v1.0 VO.ppt Quality Review Is Mandatory Pub 4012 Tab 13 Form 13614-C Section C LEVEL 2 TOPIC 11/30/2010 * NJ Training TY2010 v1.0 PowerPoint PPT presentation free to view . Quality Sleep with Leesa Mattress Reviews - Leesa …
“APQR is a Annual Product Quality Review somewhere known as APR (Annual Product Review)” APQR contains a documented evidence oriented review of all activities related to a product manufactured in a organization, it covers all parameters which affects a product quality from Manufacturing stage to market performance. Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article (PDF Available) · February 2012 with 4,656 Reads How we measure 'reads' A 'read' is counted each time
Both parts of the EU-GMP Guidelines require the Product Quality Review (PQR) to verify the consistency and appropriateness of existing processes, but also to identify product and process improvement opportunities. The FDA 21CFR 211 requires an Annual Product Review (APR) to evaluate annually the quality standards of each drug product. Periodic Product Quality Reviews or Annual Product Reviews of drug products are required by the U.S., EU and Canada. If used effectively, these reviews can be a powerful Quality Assurance tool. The Product Quality Review Process includes: Process Performance and Product Quality Monitoring, Corrective Action/Preventive Action, Change Management
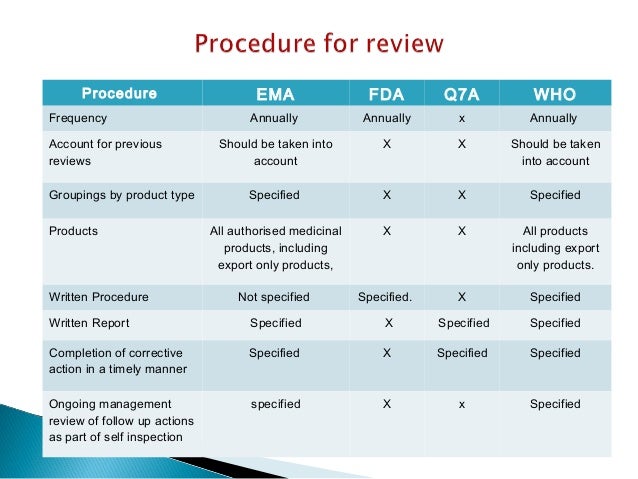
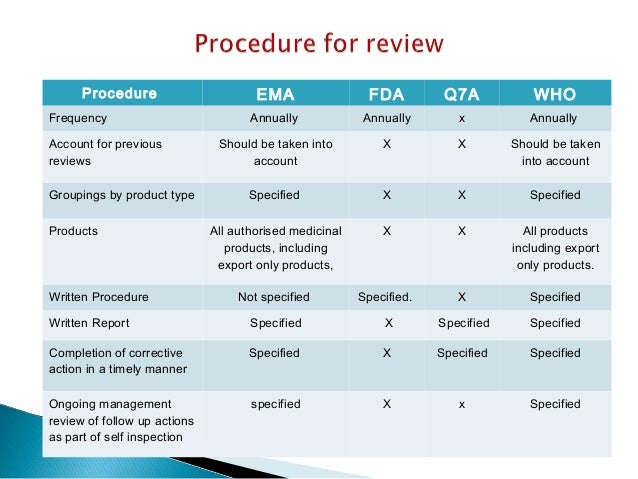
Annual Product Review Developing an SOP Presented by Steve Williams Director – SeerPharma P/L Sept 2010 . Objective FDA 211.180(e) EU/PIC/s Determine appropriateness, or need to change, product specifications Required Required Same as above for starting materials Not specified Required Need to change manufacturing procedures Required Not specified Same as above for in-process controls Welcome to Pharmaprojects’ 2017 review of trends in pharmaceutical R&D. For around a quarter of a century now, I’ve been taking an annual look at the evolution of pharma R&D, and in this article, I’ll look at how the land lies at the start of 2017. We’ll assess industry trends by examining the pipeline
SOP for Annual Product Quality Review Purpose: - This SOP gives the method of collecting data for Annual product review Responsibility:-Q. A. ManagerPrecautions: Not applicable General Condition: Annual Product review of a finished is prepared for all the batches manufactured in a year i.e. January month to December month. 01/02/2012В В· Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review More than just a regulatory requirement, an APR helps the manufacturer to understand processes and make further improvements. By Ajay Pazhayattil, Director, Quality and Regulatory Affairs, Jarvis Street Pharma Inc. Feb 01, 2012
quality product. •EU Inspectors expect discussion & evaluation of the data presented and ID of appropriate process improvements as required •The FDA “Annual product review” is intended to confirm that every batch of product released during the review … Download a .PDF of this Case Study here: Managed Service Solution for Annual Product Review (APR) Client Business Situation. Client was experiencing multiple problems with its Annual Product Review (APR) process for its externally manufactured products, which included OTC Pharmaceuticals, Cosmetics, and Medical Devices (See Note).
13/06/2014 · PowerPoint Presentation: Annual Product Quality Review(APQR) Annual Product Quality Review (APQR) must be conducted for each commercial product. An Annual schedule for completing APQR shall be prepared by Manager QA. This schedule shall established the specific product to be reviewed and the “cut off ” date for each APR Shall be 6 2011 ANNUAL QUALITY REPORT component of care, yet such treatment plans are accompanied by other core complexities, such as increased medical data, a decrease in the average length of patient stays, and an increase in severity of illness patient categorization. Though at one time appropriate, the current quality
Objective :To lay down a procedure to conduct Annual Product Quality Review for all pharmaceutical products. Scope :This Standard Operating Procedure is applicable for all products manufactured at formulation plant of Pharmaceuticals Company (Name). Responsibility Officer / Executive – QA shall Welcome to Pharmaprojects’ 2017 review of trends in pharmaceutical R&D. For around a quarter of a century now, I’ve been taking an annual look at the evolution of pharma R&D, and in this article, I’ll look at how the land lies at the start of 2017. We’ll assess industry trends by examining the pipeline
APR - Audi 2.7T Audi A6/Allroad ECU Upgrade; Select your vehicle. APR - Audi 2.7T Audi A6/Allroad ECU Upgrade. Not rated yet. Get more power and torque from your 2.7T Audi with an upgraded ECU from APR. Please sign in to add review There are no comments yet. Be the first to leave a comment Audi allroad 2.7 t review Borenore 2004 Audi Allroad Reviews: Read 10 candid owner reviews for the 2004 Audi Allroad. 2004 Audi Allroad Review . hayabusa2003 writes: Appearance: Performance: Quality: Overall: For a 2.7 liter V6, the car has get up and go. There is some Turbo lag, but it's manageable. This car has no blind spots, it's like driving in a greenhouse


